Making ATP from various foods, glycolysis, pyruvate oxidation, Krebs cycle, and the electron transport chain, and anaerobic respiration.
Cellular Energy
Organisms from every kingdom use adenosine triphosphate (ATP) as an energy supply for thousands of chemical reactions that occur in every cell. ATP consists of an adenine (one of the four nitrogenous bases), a ribose sugar, and three phosphates (Figure 4.1). Energy is transferred from the ATP when the last phosphate bond is broken, and the phosphate (inorganic phosphate, Pi) binds to another molecule; this converts the ATP into ADP (adenosine diphosphate): ATP→ADP+Pi.ATP→ADP+Pi.
When the phosphate from ATP binds to a protein, such as the sodium-potassium pump membrane protein (Chapter 3), the protein changes its conformational shape, which then aids a cellular process, such as moving sodium ions out of the cell.

Cellular Production of ATP
Our cells make ATP from the foods we eat. Recall from Chapter 2 that we eat macromolecules: proteins, complex carbohydrates (polysaccharides), and fats (lipids). When we digest these foods, they are broken down into their building blocks—amino acids, monosaccharides (sugar), and glycerol and fatty acids—and then taken up by our cells. Food energy (such as glucose) is converted into chemical energy (ATP) through the process of cellular respiration. Cells use ATP to perform thousands of functions every second. Excess food energy that is not used for ATP production becomes stored as triglycerides (fat) in the liver or adipose tissue until needed. Therefore, to maintain our body weight, we need to eat the same amount of food energy that our cells use in chemical energy.
Did You Know?
- Carbohydrates contain 4 Calories (Cal) per gram; 1 tsp of sugar is 4 g and therefore has 16 Cal.
- Protein contains 4 Cal per gram.
- Fats contain 9 Cal per gram; 1 tsp butter is approximately 4 g and therefore has 36 Cal.
- Alcohol contains 7 Cal per gram.
- 1 beer (12 oz) is approximately 150 Cal and contains alcohol, carbohydrates, and a small amount of protein.
- 1 glass of wine (6 oz) is approximately 120 Cal and also contains carbohydrates and some protein.
- 1 shot (1 oz, which is 28 g but contains water and is not 100% alcohol) is approximately 65 Cal.
Food Energy
Nutritionists measure the amount of energy in the food we eat in terms of Calories (kilocalories). A temperature of 1 mL of water 1ºC. This is a very small amount, so kilocalorie (Calorie, Cal) is used as the unit of measure. Depending on their age, body size, genetic factors, fitness level, and amount of exercise, adults require anywhere from 1500 to 3000 Cal per day to maintain their body weight (fewer calories are required as we get older).
Consuming 3500 extra kilocalories can lead to a weight gain of 0.45 kg (1 lb, same as a block of butter). If you add one can of regular cola per day (130 Cal per can) to your diet without changing your food intake or amount of exercise, you will gain 0.45 kg of body weight after approximately 23 days.
Conversion of Food Energy into ATP Molecules
Eukaryotic cells primarily use glucose to produce ATP; however, our cells can also use fatty acids, amino acids, and nucleotides (Section 4.5). Through a series of oxidation and reduction reactions the glucose is eventually completely broken down; the hydrogens move from the glucose to a coenzyme, leading to the production of ATP. The carbon-hydrogen bonds in methane are the source of energy in natural gas, just as the carbon-hydrogen bonds in glucose are used in our cells (Figure 4.2). In oxidation-reduction reactions, electrons become transferred from the nutrient being broken down, such as glucose, to the coenzyme NAD+ or FAD+; these are then reduced to produce NADH or FADH2. Oxidation and reduction reactions always go together, and are referred to as redox reactions (Figure 4.3). You can remember oxidation and reduction using the phrase “LEO the lion says GER”: Loss of Electrons is Oxidation; Gain of Electrons is Reduction.

The process of oxidizing food molecules (glucose, amino acids, fatty acids, glycerol, and nucleotides) and reducing NAD+ ions leads to the production of ATP; this process is called cellular respiration. The oxidation of glucose in the presence of oxygen is called oxidative phosphorylation or aerobic respiration. This is why we need to breathe oxygen. When the level of oxygen in a cell is temporarily low, ATP is produced through anaerobic respiration: for example, when muscle cells have low oxygen levels during strenuous exercise such as sprinting.

Stages of Aerobic Cellular Respiration
- Glycolysis occurs in the cytoplasm.
- Pyruvate oxidation occurs in the matrix of the mitochondria.
- Krebs cycle occurs in the matrix of the mitochondria.
- Electron transport proteins are found in the inner membrane of the mitochondria.
Aerobic respiration occurs in four main stages, and each stage occurs in different areas of the cell (Figure 4.4). During the four stages, one glucose molecule becomes entirely oxidized (broken down) and yields many ATP molecules and heat. The reaction equation is as follows:

Oxidization of Glucose in the Cytoplasm
Glycolysis is the process of oxidizing glucose to produce two molecules of pyruvate (Figure 4.5). Glycolysis occurs in the cytoplasm of all cells in the body in all living organisms; it is the oldest evolutionary process used to make ATP. Glycolysis—a 10-step process of chemical reactions—involves the oxidation of glucose (six carbon atoms) and the reduction of NAD+, which results in the production of two pyruvate molecules (three carbon atoms each). Once glucose enters the cell (see coupled transport, Chapter 2), it is phosphorylated with two phosphates from two ATP molecules; therefore, two ATP molecules are required to start glycolysis.

Once glucose is phosphorylated, oxidation-reduction (redox) reactions produce two three-carbon pyruvate molecules. During this process, two NAD+ molecules are reduced to NADH, and four ATP molecules are formed (a process called substrate-level phosphorylation). Since two ATP molecules are required to begin the process, a net amount of two ATP molecules are produced during glycolysis. The NADH molecules are used by the electron transport chain to produce many more ATP molecules.
Pyruvate Oxidation
The end result of glycolysis is two pyruvate molecules, each containing three carbons. These pyruvate molecules can diffuse through the mitochondrial membrane into the matrix, where further oxidation can occur. Pyruvate oxidation results in electrons and hydrogen ions moving from pyruvate to the electron acceptor NAD+ to produce more NADH molecules (Figure 4.6). Also, during this process, a carbon atom is removed from each pyruvate, which leaves a two-carbon molecule (an acetyl group) that combines with coenzyme A. The final product is a molecule called acetyl-Coenzyme A (acetyl-CoA). The enzyme that removes the carbon atom from pyruvate is called pyruvate dehydrogenase. The carbon atom is removed as a carbon dioxide (CO2) molecule. The process of removing this carbon as CO2 is called decarboxylation. This explains why we exhale carbon dioxide.

Cellular ATP Capacity
Depending on what cell functions are occurring at a particular time, cells require more or less ATP. During exercise, for example, muscle cells require very high levels of ATP for muscle contraction; however, during periods of rest, those cells do not need to make as much ATP. All cells use ATP for many chemical reactions, including moving substances into or out of the cell, producing proteins, transporting substances within the cell, producing new organelles, breaking down and recycling old organelles, replicating DNA, and facilitating mitosis. Muscle cells require the most ATP for contraction, which is why exercise is the primary way to burn calories. Cells store extra nutrients as fat that can be broken down later when more ATP is needed. When fats are used as energy, they do not go through glycolysis, they go through a process called beta-oxidation. Because they are long carbon-hydrogen chains, fats can be converted into two-carbon acetyl groups (Section 4.5). Acetyl-CoA, formed in combination with coenzyme A, enters the Krebs cycle (the next stage of the cellular respiration process).
Did You Know?
B vitamins are essential for our cells to make energy. NAD+ stands for nicotinamide adenine dinucleotide, formed from the vitamin B3, niacin. FAD+ is flavin adenine dinucleotide, formed from the vitamin B2, riboflavin. And coenzyme A is produced from vitamin B5, pantothenic acid. A deficiency in B vitamins leads to extreme fatigue because not enough ATP is produced. Foods high in B vitamins include meat, eggs, nuts, seafood, and yogurt.
Krebs Cycle
The Krebs cycle involves a series of nine reactions.
1. Acetyl-CoA enters the cycle and binds to a four-carbon molecule, oxaloacetate, forming a six-carbon molecule, citrate, also called citric acid. Because of the formation of this molecule, the Krebs cycle is also called the citric acid cycle (Figure 4.7).
2. The two-carbon molecules are removed as carbon dioxide. Recall from the initial equation that six CO2 molecules are formed for each glucose molecule that is oxidized: two are formed when pyruvate is oxidized, and the other four are formed at this point during the Krebs cycle. During the process of decarboxylation (removal of carbon to produce CO2), electrons and hydrogen ions are transferred to NAD+, forming NADH and FADH2. Since two carbons have been removed as CO2, in the third step one four-carbon molecule remains: oxaloacetate. This is the initial starting material, which is regenerated and ready for the next cycle.
3. For each acetyl-CoA that enters the Krebs cycle, one molecule of ATP is produced. Since two molecules of acetyl-CoA enter Krebs, two molecules of ATP are produced.
The most important result of the Krebs cycle is the production of the six NADH and two FADH2 molecules. These reduced coenzymes are important because they carry the electrons and hydrogen ions that are required for the electron transport chain to produce a significant amount of ATP.

ELECTRON TRANSPORT CHAIN AND CHEMIOSMOSIS
Importance of NADH and FADH2
The coenzymes NAD+ and FAD—reduced to form NADH and FADH2 during the processes of glycolysis, pyruvate oxidation, and Krebs cycle—provide the necessary electrons and hydrogen ions (protons) for the electron transport chain. Recall from Chapter 3 that proton pumps are membrane proteins that move hydrogen ions across a membrane. The proton pump membrane proteins are located in the cristae—the inner layer of the mitochondrial membrane. These hydrogen ions move across the inner mitochondrial membrane to form a H+ gradient in the intermembrane space (Figure 4.8). It is important to have a high concentration of hydrogen ions in the intermembrane space because the concentration difference is used as energy for the production of ATP (secondary active transport). Because molecules always go toward equilibrium (Chapter 3), the high concentration of H+ in the intermembrane space makes the H+ ions “want” to move down the concentration gradient and back into the matrix of the mitochondria. The only way these H+ can move back into the matrix is through a specific membrane protein called ATP synthase. Chemiosmosis is the term used to describe the process of using a chemical (H+) gradient for moving across the membrane, with the result of producing many ATP molecules. The enzyme activity of ATP synthase phosphorylates ADP to form ATP.
Electron Movement through Membrane Proteins
When NADH and FADH2 split so that the H+ can be pumped into the intermembrane space, the electrons are transferred through the membrane proteins (hence the term, electron transport chain). Recall from Chapter 3 that active transport requires energy to move molecules toward an area of higher concentration. The movement of H+ ions into the intermembrane space is active transport, and the energy to move the H+ ions comes from the transfer of electrons through the electron transport proteins (Figure 4.8).

Electrons at the End of the Electron Transport Chain
After being transported through the membrane proteins, the hydrogen ions and the electrons need to combine with something—oxygen. If they didn’t, they would build up in the matrix of the mitochondria, the electrons would react with other molecules, and the protons would produce acidic conditions. The final electron acceptor in our cells is oxygen. This is why we need to breathe. The electrons and the protons (H+) that have moved through the electron transport chain and ATP synthase combine with oxygen to form water molecules (see Figure 4.9).

OTHER NUTRIENTS THAT PRODUCE ATP
Our cells primarily use glucose as the starting material for ATP production, but most cell types can also produce ATP from fatty acids, glycerol, amino acids, and nucleotides (Figure 4.10). Glucose is the only nutrient that undergoes glycolysis to produce pyruvate. Other nutrients enter the process at various stages.

When eaten, proteins are digested into their building blocks: amino acids. Amino acids can be used to produce NADH and FADH2 molecules through oxidation, which leads to ATP production. Amino acids contain nitrogen groups (amino groups, see Chapter 2), that have to be removed through a process called deamination. The nitrogen groups are removed as ammonia (NH3), which is then converted to urea and excreted by the kidneys. The deaminated amino acids can then be converted into pyruvate or acetyl-CoA, which will become oxidized.
Nucleic acids, found in all foods made of cells— vegetables and meats—are digested into their building blocks, nucleotides, which can also be used to produce ATP. Nucleotides also contain nitrogen, and since nitrogen is not used to make energy, it must be removed. Nitrogen removed from pyrimidine nucleotides is converted into urea, and nitrogen from purines is converted into uric acid; both are excreted in the urine by the kidneys.
Fats stored in adipose tissue, muscles, and the liver are triglycerides and can be used to make large amounts of ATP. Lipase is the cell enzyme that breaks the bonds between the fatty acids and the glycerol; this involves a hydrolysis reaction called lipolysis. Fats in adipose tissue are carried through the bloodstream by high density lipoproteins (HDLs) to cells—mostly the muscle cells—that need fat for energy. A high level of blood HDLs is associated with a decreased risk of heart disease because it is an indication that the body is breaking down stored fat. Fatty acids can also be carried to the muscle cells by the plasma protein albumin. Glycerol can be converted into pyruvate.
Since fatty acids are long chains of carbon-hydrogen bonds, they can be oxidized into two-carbon acetyl-CoA molecules and then enter the Krebs cycle. This process is called beta-oxidation (Figure 4.11). Recall that fatty acid chains can be formed from acetyl-CoA if ATP levels are sufficient in the cell and if excess nutrients are present.

Did You Know?
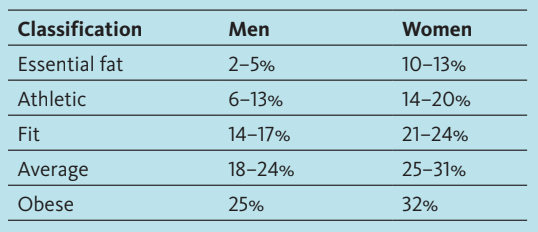
Healthy body fat percentages for males are quite different from females, as shown in this chart based on data from the American Council on Exercise.
ATP Production without Oxygen
In some situations, certain body cells may temporarily lack oxygen: for example, during sprinting when muscle contraction requires bursts of energy. The muscle cells quickly deplete oxygen during strenuous, high-intensity activity, and the cell relies only on glycolysis for ATP production. Recall that a small amount of ATP is produced through substrate-level phosphorylation in the cytoplasm during glycolysis. When oxygen is absent, ATP is produced through anaerobic respiration. The small amount of ATP produced means that fatigue happens very rapidly. Through glycolysis, it is possible to produce enough ATP for 95 to 100% effort for 20 to 30 seconds before fatigue prevents further muscle contraction. Endurance activities such as hiking use aerobic respiration, and approximately 60 to 70% of maximum effort can last for more than an hour (depending on fitness level) before muscle fatigue occurs.
Since oxygen is required as the final electron acceptor in the electron transport chain, when oxygen is absent the electron transport process cannot occur. Therefore, the pyruvate produced during glycolysis is not oxidized to produce acetyl-CoA; instead, the three-carbon pyruvate is converted into a three-carbon lactate, also called lactic acid. You can feel the burning sensation of lactic acid in your muscles during high-intensity anaerobic exercise. The NADH molecules formed in this process must be converted back into NAD+ because the H+ is not used to build a proton-concentration gradient. The process of moving the H+ from NADH to pyruvate to form lactate is called fermentation. Fermentation in humans can be summarized by the following equation:
pyruvate+NADH→lactate+NAD+pyruvate+NADH→lactate+NAD+
Once oxygen is again available, the lactate can be converted back to pyruvate, and aerobic respiration can resume.
Anaerobic Respiration in Yeast
In yeast, the three-carbon pyruvate goes through a decarboxylation step, where a carbon is removed as carbon dioxide, producing acetaldehyde. Then the H+ from NADH is transferred to acetaldehyde, forming a two-carbon ethanol molecule. Ethanol is the alcohol molecule found in wine and beer (Figure 4.12). The production of CO2 by yeast makes it a useful organism for baking bread; the CO2 makes the bread fluffy, and the ethanol evaporates during baking (Figure 4.13).

REGULATION OF METABOLISM
Since muscle cells require more ATP than any other cells in the body, we can stimulate the breakdown of stored fat with exercise. Slow-twitch muscle fibres preferentially break down fatty acids for energy during aerobic, endurance exercise (Chapter 14). Fast-twitch fibres preferentially use glucose (and break down glycogen) for energy during anaerobic, high-intensity exercise; however, this type of exercise still stimulates fat breakdown because the liver converts fat into glucose to supply these cells (gluconeogenesis). Therefore, any type of exercise will stimulate fat breakdown in some way. The longer the duration or the higher the intensity, the more total calories will be burned.
The following hormones affect fat storage or promote fat breakdown:
Insulin—signals cells to take up blood glucose and convert it into fat or glycogen for storage. Eating a meal causes blood sugar to increase, which stimulates the production of insulin, which causes blood sugar levels to decrease.
Glucagon—signals the liver to break down glycogen and fat to produce glucose. This process, called gluconeogenesis, occurs during periods of fasting or exercise and causes blood sugar levels to increase.
Epinephrine, norepinephrine, and cortisol—is produced during a stress response, fasting, and during exercise to stimulate the breakdown of glycogen and fat to increase blood sugar levels. Cortisol also causes the up-regulation of receptors that bind epinephrine and norepinephrine and therefore act synergistically during a stress response.
Growth hormone—is produced during deep sleep, periods of fasting, and exercise to stimulate the breakdown of fat and increases blood sugar.
DHEA (testosterone-like hormones)—are produced by the adrenal cortex during stress and exercise to stimulate the breakdown of fat to increase blood sugar. DHEA also stimulates the production of growth hormone, which is required for protein synthesis during and after exercise to repair muscle cells.
Thyroid hormones (T3 and T4)—bind to receptors in the cell nucleus and regulate the expression of genes that promote cellular respiration and utilization of oxygen. Thyroid hormones are required for the metabolism of carbohydrates, fats, and protein.