I thought it would be a good idea to explain what an alkaline diet is and how pH is regulated in the body and then you can make your own decision about it.
According to the alkaline diet there are certain minerals that are acidic or alkaline - acidic foods lead to disease whereas alkaline foods prevent disease.
Minerals that form acids include Sulfur, which forms sulfuric acid (H2SO4), Phosphorus, which forms phosphoric acid (H3PO4) and nitrogen, which forms nitric acid (HNO3).
Alkaline minerals are Ca (calcium), K (potassium), and Mg (magnesium) – they can’t form acids. So even though lemons contain citric acid, they are considered an alkaline food because they are high in potassium, calcium, and magnesium.
Acid-forming foods | Alkaline foods |
· Meat · Dairy · Carbonated drinks (high in phosphate) · Fruit juices · Roasted nuts and peanuts · Grains/ pastries · Alcohol · Fish · Eggs · Fast food · Processed food · Sugar and artificial sweeteners | · Fruit · Vegetables · Legumes · Olive oil · Raw nuts · Herbs |
Can our diet impact the pH in our body?
First, it is important to understand a little chemistry
We measure the acidity or alkalinity (an alkaline substance is also called a base) of any substance by pH, the scale goes from 0 (very acidic) to 14 (very basic), with 7 being neutral. Here is a pH scale showing where some common substances fall.
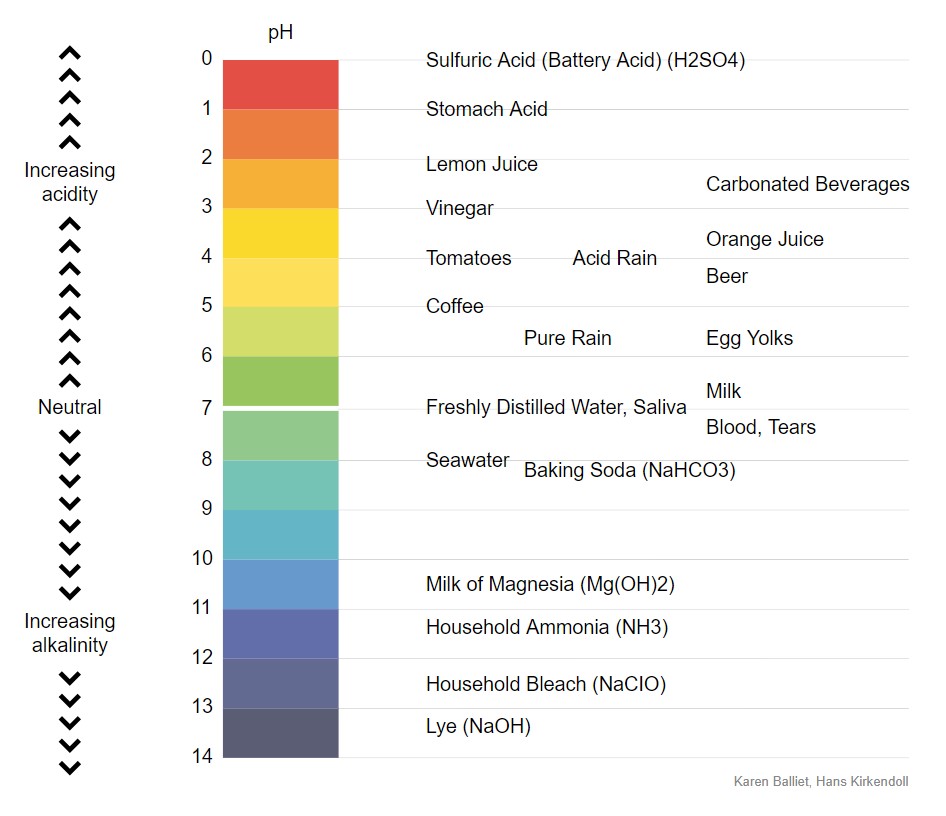
I want you to note where blood is on the scale. Our blood pH is very tightly regulated to be between 7.35-7.45 (blood in veins is slightly closer to 7.35 because veins carry more carbon dioxide than arteries), so, blood is slightly alkaline. If your blood pH goes below 7.35, it is called acidosis and you would vomit because that is that fastest way to get rid of a lot of excess acid, and you would feel out of breath; breathing rate and depth would increase because breathing off carbon dioxide will increase blood pH. Severe acidosis causes your brain to malfunction and you would go into a coma and die. If your blood pH goes above 7.45, it is called alkalosis and would cause severe weakness, light-headedness, numbness or tingling, possibly muscle spasms, and in severe cases your brain would malfunction and you would go into a coma and die.
Fun fact: People can vomit when doing extremely intense exercise because lactic acid made by the muscles lowers blood pH and vomiting helps your body increase pH very quickly.
The kidneys and the lungs are the primary organs that regulate blood pH and deal with the small fluctuations that happen during normal metabolism. When pH becomes acidic (closer to 7.35) then the kidneys excrete Hydrogen ions (acid) and the lungs increase respiration to get rid of CO2. When the blood becomes alkaline (closer to 7.45) the kidneys excrete bicarbonate and breathing slows.
Fun fact: If you hold your breath you retain CO2 and increase the acidity of your blood and if you hyperventilate then you can make your blood alkaline from breathing off too much CO2. Our brainstem regulates this though and won’t let you alter your pH by very much. If you hold your breath for too long, your brainstem will force you to breath once you blood pH gets too acidic; if you hyperventilate then at some point you would faint and hyperventilation stops. Our body is quite protective of our blood pH.
pH in different parts of the digestive tract
Notice in the pH scale above that stomach acid is between 1.35 – 3.5. Food is first broken down in our acidic stomach. The stomach produces hydrochloric acid when we eat and this serves a few functions:
- Activates pepsinogen to become pepsin, which is an enzyme that breaks down protein. Only protein is chemically digested in the stomach, fats and carbs are just mechanically broken down in the stomach and then chemically digested in the next step, the small intestine.
- Stomach acid kills any bad microbes we ingest
- Stomach acid signals the release of hormones that signal other parts of the digestive tract, such as the pancreas to release its contents into the small intestine
Digestion in the small intestine must take place in alkaline conditions. The enzymes released by the small intestine and pancreas to break down fat, carbs, and the rest of the protein, only function at an alkaline pH. The small intestine becomes alkaline because the pancreas releases sodium bicarbonate. See the pH scale above, bicarbonate is baking soda, and has a pH of about 8.3-9.0. So no matter how much citric acid or acetic acid (vinegar) you eat, or stomach acid you make, the pancreas is going to neutralize it with bicarbonate once it reaches the small intestine.
Also, different regions of the body have a different pH for various reasons. Urine pH will fluctuate the most because it is buffering the blood, so if the blood pH decreases, the urine will be more acidic, if blood pH increases then urine will be less acidic (assuming you don’t have kidney disease). Here is a great chart comparing the pH of different body regions.
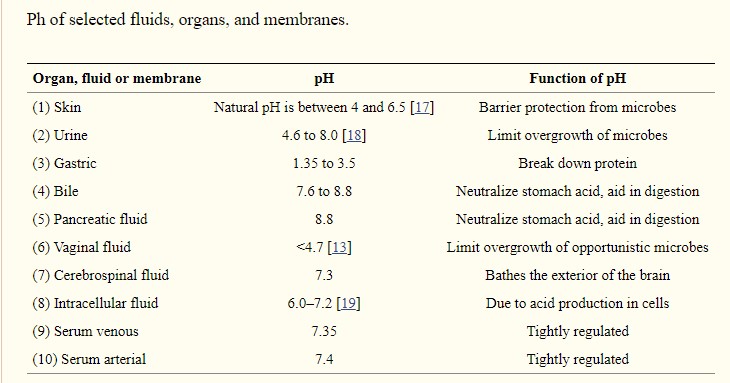
The pH inside our cells (intracellular fluid in chart above) is a bit more acidic than our blood; it can fluctuate between 6.0 – 7.2 and this is due to acidic molecules produced by the cells for various reasons, including normal metabolism, such as making energy (ATP) and producing carbon dioxide or lactic acid, or production of waste products from breaking down cellular components. If acids are produced, then they move out of the cells and into the blood where they are excreted by the kidneys or breathed off as CO2 by the lungs.
The pH of our urine can fluctuate more than our cells or our blood, between 4.6 – 8.0, 4.6 being quite acidic but some acidity is necessary for preventing the growth of bacteria in the urinary tract. One common home remedy for a urinary tract infection (UTI) is to consume a lot of cranberry juice, this helps because it is acidic and the acidity of the urine produced kills bacteria in the urinary tract. FYI, consuming a lot of ascorbic acid (vitamin C) would work too.
If you use pH strips to test the acidity of your urine you can see how much acid or base your kidneys are regulating but it does not tell you the pH of your blood or your intracellular fluid.
I think it is necessary to understand what an acid and a base is, so here are 2 simple examples:
- Hydrochloric acid is made of a hydrogen ion (H) and a Chloride ion (Cl). When the H ion separates from the Cl then it is an acid, free H ions are acidic and lower pH.
- A base is something that can take up a H+ ion. An example is bicarbonate (HCO3–) forms carbonic acid.
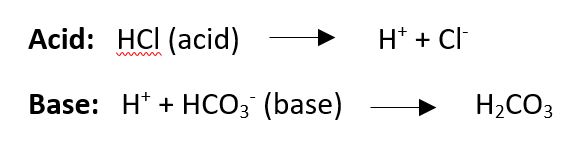
Buffers in the body regulate pH by taking up H+ or releasing them, this keeps pH very stable
There are a few key buffering systems in the body. One is the carbonic acid – bicarbonate system mentioned above, that base reaction can move in the opposite direction to release H+ ions if the pH became too high, releasing the H+ ions brings the pH back down.
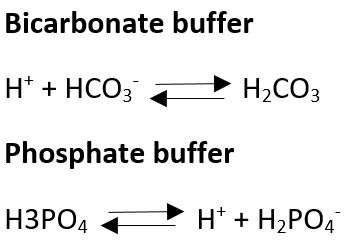
Another system is the phosphate system. H+ ions can be added or removed from phosphate (PO4) to keep pH stable. The alkaline diet states that meat is high in phosphates and they are acidic but in the body they are used for various reasons, making cell membranes and DNA, but they also buffer pH.
Lastly, amino acids can buffer pH. Amino acids are the building blocks of all proteins. Amino acids have a nitrogen group that can take up H+ and acts like a base, and the COOH (carboxyl group) can lose a H+ and acts like an acid, so an amino acid is technically an acid and a base.
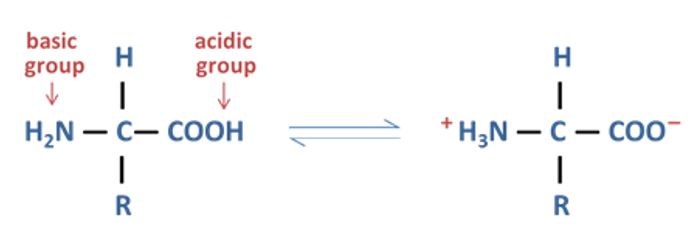
Meat is considered acidic because it contains phosphorus, but plants also contain nitrogen and phosphorus so how did the classification of these foods come about?
If you burn foods until there is just ashes left, all that is left is minerals. If you test the pH of the ashes of meat, dairy, nuts, and grains, they have a lower pH, so the combination of all the minerals leftover is acidic. If you do the same with fruit and vegetables, the ash pH is alkaline.
The question is whether or not the pH of minerals in ash translates to an effect on the pH in our cells. For example, the 2 amino acids that contain sulfur are methionine and cysteine and there are more of those amino acids in meat protein than plant protein, making meat ash more acidic than vegetable ash. But sulfur amino acids are very important for cellular function and the presence of sulfur amino acids in the cells does not mean that sulfuric acid will form. Methionine is essential for cellular function and regulating metabolic processes.
Another factor to consider is reasons why acidosis can occur in the body
- Kidneys are not removing enough H+ ions from the blood – chronic kidney disease (CKD), most common cause of kidney damage is high blood pressure/ cardiovascular disease
- Diabetic acidosis can occur is ketones build up in the body (ketoacidosis)
- Severe diarrhea – excessive loss of sodium bicarbonate
- Severe dehydration
- Lactic acid build-up
- Excessive alcohol consumption
- Cancer
- Intense exercise
- Taking too much aspirin (salicylates)
- Lack of oxygen from a heart attack, stroke, or ischemia (cut off blood flow for whatever reason)
- Seizures
Diet simply cannot impact blood pH.
The proponents of an alkaline diet most commonly say that the main benefits are bone health and cancer prevention. The research is mixed, some studies say there are benefits and others say there is not.
Perhaps the benefits seen in some studies occur because people following an alkaline diet are eating more plant foods rather than any impact on pH??
Below is a list of conclusions from various research papers about the effectiveness of an alkaline diet for various health conditions
The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health?
- There is no substantial evidence that an alkaline diet improves bone health or protects from osteoporosis
- Increased fruit and vegetables in the diet improve the potassium/ sodium ratio that improves bone health, reduces muscle wasting, and may mitigate chronic diseases such as hypertension and strokes
- Increased growth hormone in alkaline diet may improve cardiovascular health, memory and cognition
- Increased magnesium is beneficial because it is required for the function of many enzymes and activates vitamin D
- An alkaline diet may be beneficial for people on chemotherapeutic agents
Dietary acid load is not associated with lower bone mineral density except in older men
- With the possible exception of older men, dietary acid load does not have a measureable effect on bone health regardless of total calcium intake
- Men with low dietary calcium showed that renal acid load had an adverse effect on bone health and highlights the importance of calcium intake. The same interaction was not observed in women.
- Acid-forming diets have no impact on calcium levels in the body
Meta‐Analysis of the Effect of the Acid‐Ash Hypothesis of Osteoporosis on Calcium Balance
- There is no evidence from superior quality balance studies that increasing the diet acid load promotes skeletal bone mineral loss or osteoporosis. Changes of urine calcium do not accurately represent calcium balance. Promotion of the “alkaline diet” to prevent calcium loss is not justified.
- A causal association between dietary acid load and osteoporotic bone disease is not supported by evidence and there is no evidence that an alkaline diet is protective of bone health
- The original research regarding diet and acid load stemmed from studies on patients with kidney disease and was then extrapolated to the healthy population converted a hypothesis into nutritional recommendations for preventing osteoporosis. Food does not influence pH in healthy people.
Acid treatment of melanoma cells selects for invasive phenotypes
- Cancer cells grow faster in an acidic environment but the cancer cells themselves produce the acid, the environment does not cause the production of cancer cells.
Is an alkaline diet better for you? Canadian cancer Society
- There is no evidence to support the claims that an alkaline diet will help you lose weight, increase energy, reduce the risk of heart disease, or reduce the risk of cancer
Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women
- Low grade chronic metabolic acidosis exists normally in humans eating ordinary diets that increase net rates of acid production and this increases with age and contributes to a loss of muscle mass
- Orally administering potassium bicarbonate (to reduce acid load) reduced loss of nitrogen in the urine and reduced blood hydrogen ion concentration in post-menopausal women (urinary ex creation of nitrogen is an indication of muscle
Alkaline minerals reduces lower back pain
- An alkaline mineral supplement reduced back pain in patients with chronic lower back pain
Other References used in this article
Determinants of blood pH in health and disease
U.S. National Library of Medicine – Metabolic Acidosis
Open source Anatomy and Physiology


Great article! I love the “fun facts”.
That was a great classroom.
Understanding the basics of our bodies is highly important.
This was well broken down. Chemistry was not my forte 🙂