The chemistry of water, body water compartments, dehydration synthesis and hydrolysis reactions to make and break macromolecules.
WATER
Universal Solvent
All living things from the smallest single-celled bacterium to multicellular plants and animals are composed of anywhere from 45 to 95% water. In humans, infants are approximately 75% water, and this amount decreases with age: mostly in the first 10 years of life, but continuing into old age. Healthy adult males are on average 60% water, and females are 55% water. Healthy females compared to healthy males of appropriate body weight have slightly less water because they tend to carry more body fat. Anyone who is obese can have as little as 45% body water content. One litre of water weighs 1 kg. An average 70 kg male (154 lb) has approximately 42 L (60%) of body water. Body water is distributed among three main compartments: one intracellular and two extracellular.
- Intracellular—40% of body weight (approximately 28 L)
- Extracellular—20% of body weight (approximately 14 L)
a. Interstitial fluid—13% (approximately 9 L)
b. Blood plasma—7% (approximately 5 L)
Many important nutrients, ions, and molecules are dissolved in the water inside and outside of cells. Water is the fluid of the body, the solvent, and it contains dissolved molecules, the solutes. Due to its unique properties, water is the universal solvent in all living things.
Did You Know?
Losing just 1% (approximately 400 mL) of your normal water content will stimulate thirst. During extensive exercise you can lose 1 L of water per hour by sweating and exhaling water vapour. Lost water comes from the extracellular compartments. Most people can tolerate losing 3 to 4% of total body water without experiencing major symptoms. A loss of 5 to 8% can cause intense thirst and physical and mental fatigue. Death can occur with a water loss of 15 to 25%.
Polarity
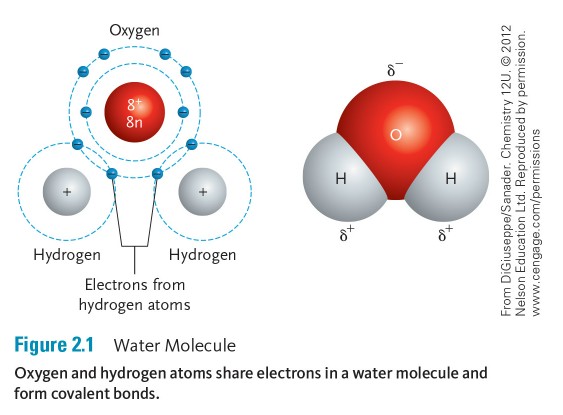
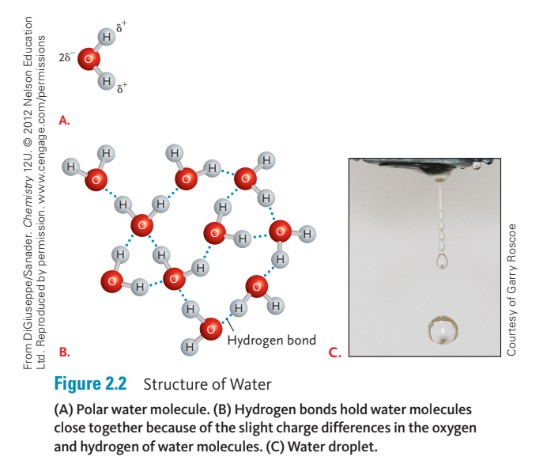
Oxygen has eight positively charged protons compared to hydrogen’s single proton. Oxygen and hydrogen form a covalent bond in which negatively charged electrons are shared between the two atoms. With the formation of the covalent bond, the negatively charged electrons are pulled slightly closer to the protons in the oxygen atom, giving the oxygen atoms a slightly negative charge and the hydrogen atoms a slightly positive charge (Figure 2.1). This charge difference causes water molecules to be polar. Because of this polarity, water molecules form hydrogen bonds, where the slight positive and slight negative charges attract (Figure 2.2). Hydrogen bonds cause water molecules to form droplets and stay close together rather than spread apart; this is called surface tension.
Hydrophilic and Hydrophobic Molecules
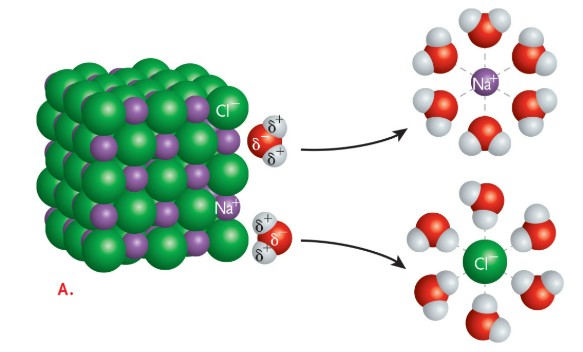
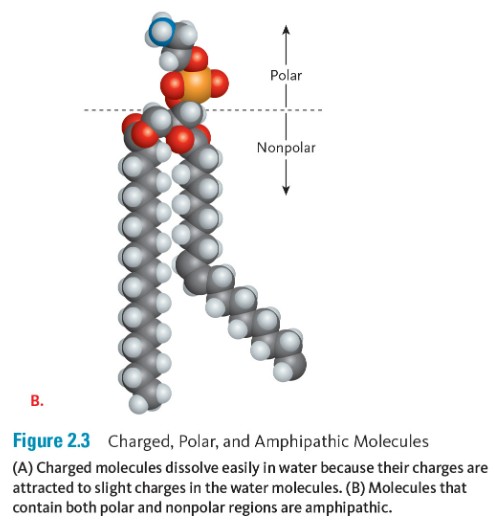
Hydrophilic means “water loving.” Any molecule or atom that has a slight charge or a full charge, such as a sodium ion or a glucose molecule, which is polar, can dissolve in water (Figure 2.3a). Substances such as oils that do not have a charge are nonpolar, and they cannot dissolve easily in water; these molecules are hydrophobic, which means “water fearing.” Both hydrophilic and hydrophobic molecules are essential to the specific functioning of cells, the formation of cell membranes, and the ways that nutrients and wastes move across those membranes. Sometimes molecules can have regions that are polar and regions that are nonpolar—such as soap, or the phospholipids in cell membranes. These molecules are called amphipathic because they have both hydrophilic and hydrophobic components (Figure 2.3b).
MACROMOLECULES
All living things are based on four primary atoms— carbon, oxygen, hydrogen, and nitrogen—that combine to form molecules. Macromolecules are very large molecules made up of combinations of many smaller molecules; for example, protein macromolecules are composed of many amino acids. Molecules combine to form macromolecules by a process called dehydration synthesis. The four macromolecules that make up all living cells are proteins, carbohydrates, nucleic acids, and lipids. These are all formed from the combination of the small individual molecules of amino acids, monosaccharides, nucleotides, and fatty acids. There are many different kinds of proteins, carbohydrates, lipids, and nucleic acids in different organisms. The proteins in a fish or an almond are different from the proteins formed in our cells after digestion and absorption, but the individual amino acids that a protein is made up of are the same in every living thing. The differences are in how those small molecules combine together. We will look at each macromolecule in more detail in the following sections.
When we eat food, we eat the polymers that plants or animals produced. These are called organic molecules because they come from living organisms. Our digestive system breaks these down into monomers (building blocks) that are absorbed into the bloodstream and circulated to the cells. Our cells use these molecules either to produce energy in the form of ATP (adenosine triphosphate) (Chapter 4) or to build human proteins, carbohydrates, nucleic acids, and lipids, which all have a multitude of cellular functions in various body systems.
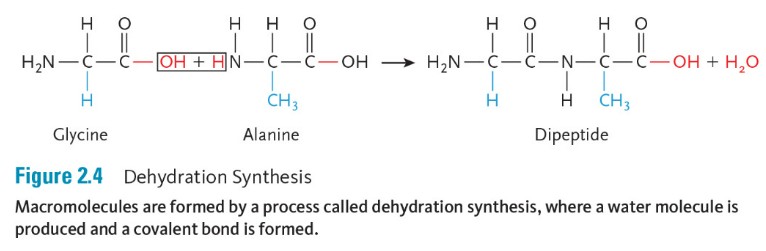
Molecules combine by the removal of a water molecule, hence the term, dehydration synthesis. For example, during protein synthesis in our cells, the protein is the macromolecule, and it is made up of amino acids. A hydroxyl group is removed from one amino acid, and hydrogen is removed from the other amino acid, and then a covalent bond forms (Figure 2.4).
When polymers are broken down in our cells or in our digestive system, the reaction known as hydrolysis occurs, which is the reverse of dehydration synthesis. A water molecule is added with the aid of a hydrolytic enzyme, and the covalent bond is broken. Hydrogen and a hydroxyl group are added to form the individual monomers (Figure 2.5).